- New call for projects for treatments that could be re-purposed to treat Epidermolysis Bullosa (EB), a life-changing and incurable rare skin disease
- Priority focus on treatments for chronic inflammation and non-healing wounds, and resulting fibrosis and skin cancer
- Joint initiative is part of LifeArc’s Rare Disease Translational Challenge – investing more than £100 million to help address some of the major obstacles facing rare disease translational science
LifeArc, the self-funded, non-profit, medical research organisation and charity, and DEBRA Austria, a patient organisation charity, are partnering to commit £2.5 million to fund projects to repurpose therapies that can be brought rapidly to patients suffering with Epidermolysis Bullosa (EB). Applications are invited from research groups at academic institutions or hospitals worldwide. There will be a priority focus on CIF (chronic inflammation, fibrosis and cancer initiation) and projects should involve therapies that can be repurposed or repositioned, and based on a strong non-clinical data package with a clear path to clinical development.
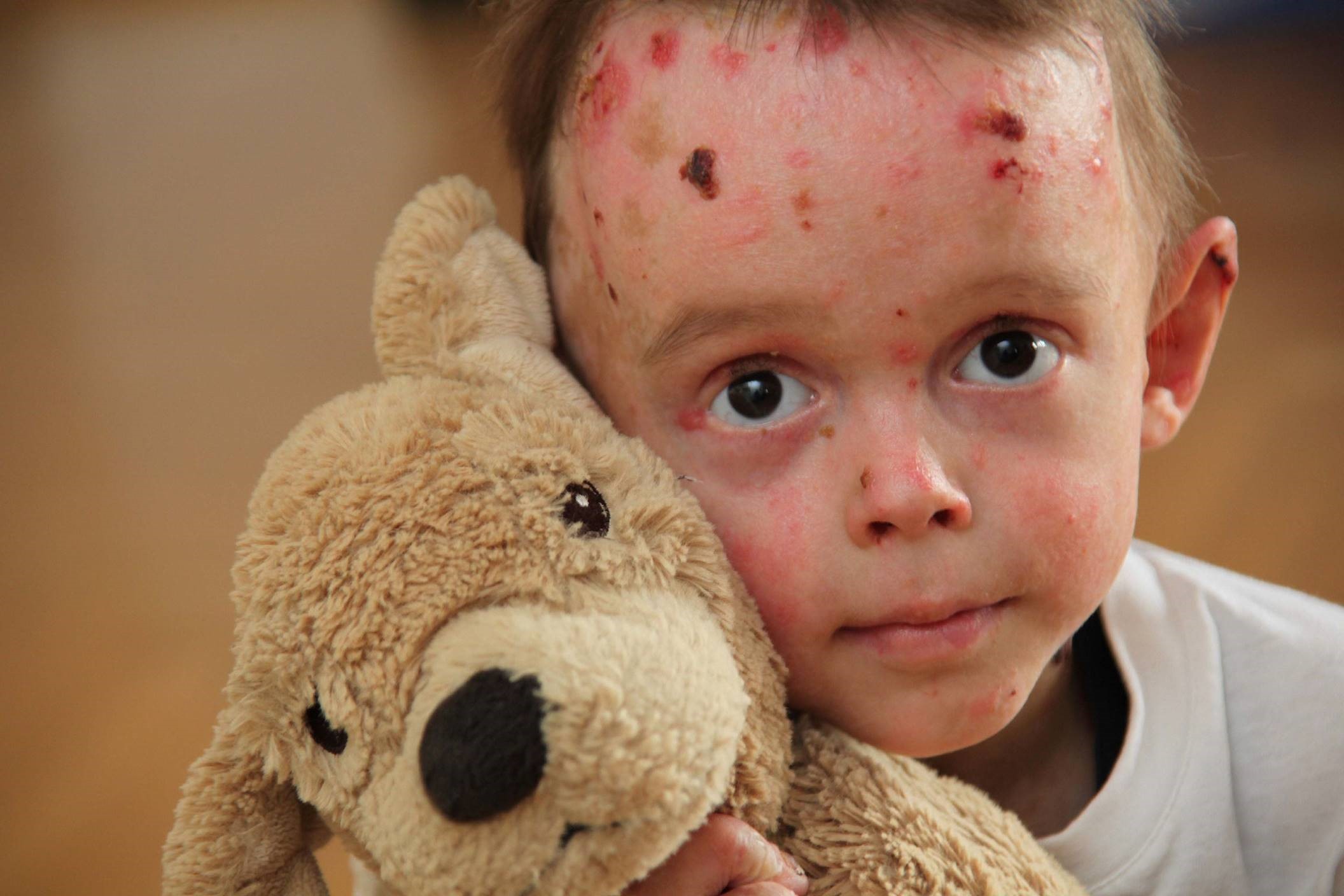
EB is a group of rare inherited disorders where the integrity of the skin and internal mucous membranes is severely disrupted, leading to chronic tissue damage, a persistent inflammatory response, and fibrosis. Progressive systemic disease can result in multi-organ complications with life-limiting consequences and reduced quality of life, and aggressive squamous cell carcinoma may initiate in chronically inflamed, non-healing wounds.
Catriona Crombie, Associate Director Technology Transfer at Life Arc, said: “This initiative is part of LifeArc’s Rare Disease Translational Challenge. Our translational challenges are long-term, collaborative research programmes shaped by what patients tell us they need and designed to tackle complex health issues by taking the best scientific ideas out of the lab and helping to turn them into medical breakthroughs that can be life-changing. While there has been encouraging progress in the development of gene and cell therapies targeting the underlying defects causing EB, there remain a number of serious consequences of the disease such as chronic inflammation and non-healing wounds that can lead to fibrosis and skin cancer. We hope this funding will help unlock the potential of existing therapies that can be repurposed or repositioned to accelerate the development of new treatments for young people living with EB.”
Rainer Riedl, Managing Director DEBRA Austria, commented: “Children born with EB are often called ‘butterfly children’ because their skin seems as fragile as a butterfly wing, leaving them susceptible to infection and disease that can severely reduce their quality of life. In all our research funding efforts, the patient has to be at the centre. We are excited to be working with LifeArc on this £2.5 million funding programme and look forward to supporting the development of new treatments for this serious disorder.”
Gaston Sendin, DEBRA Austria Research Manager, said: “Our primary goal in driving DEBRA Austria’s research agenda is to support new therapeutic approaches and push their clinical translation. We believe that repurposing may unlock new opportunities and help us bring new treatments to patients as quickly as possible.”
The call seeks innovative proposals to progress therapies where there is good evidence that they can be repurposed or repositioned to deliver increased resolution of non-healing wounds, prevention and/or reduction of chronic inflammation, inhibition and/or reduction of fibrosis, or decreased risk of squamous cell carcinoma initiation in patients with EB. Projects should seek to develop therapies that have been developed and tested for other diseases but have strong arguments for them having potential to deliver benefit in EB. The projects should take advantage of data that is already available on how the drug works, that shows how safe the drug is and at what dose it would need to be given. This should provide the basis for these drugs to progress rapidly to testing in people with EB.
The funding round will be organised in a two-stage application process. The expression of interest (EOI) stage opens on 10 July 2023 and closes on 10 September 2023. Shortlisted EOI applicants will be invited to submit full applications between 4 October and 4 December. Successful applicants will be notified in March 2024 with projects expected to begin in Q2 2024.
Media contact
Hannah Severyn
Head of Media and PR at LifeArc