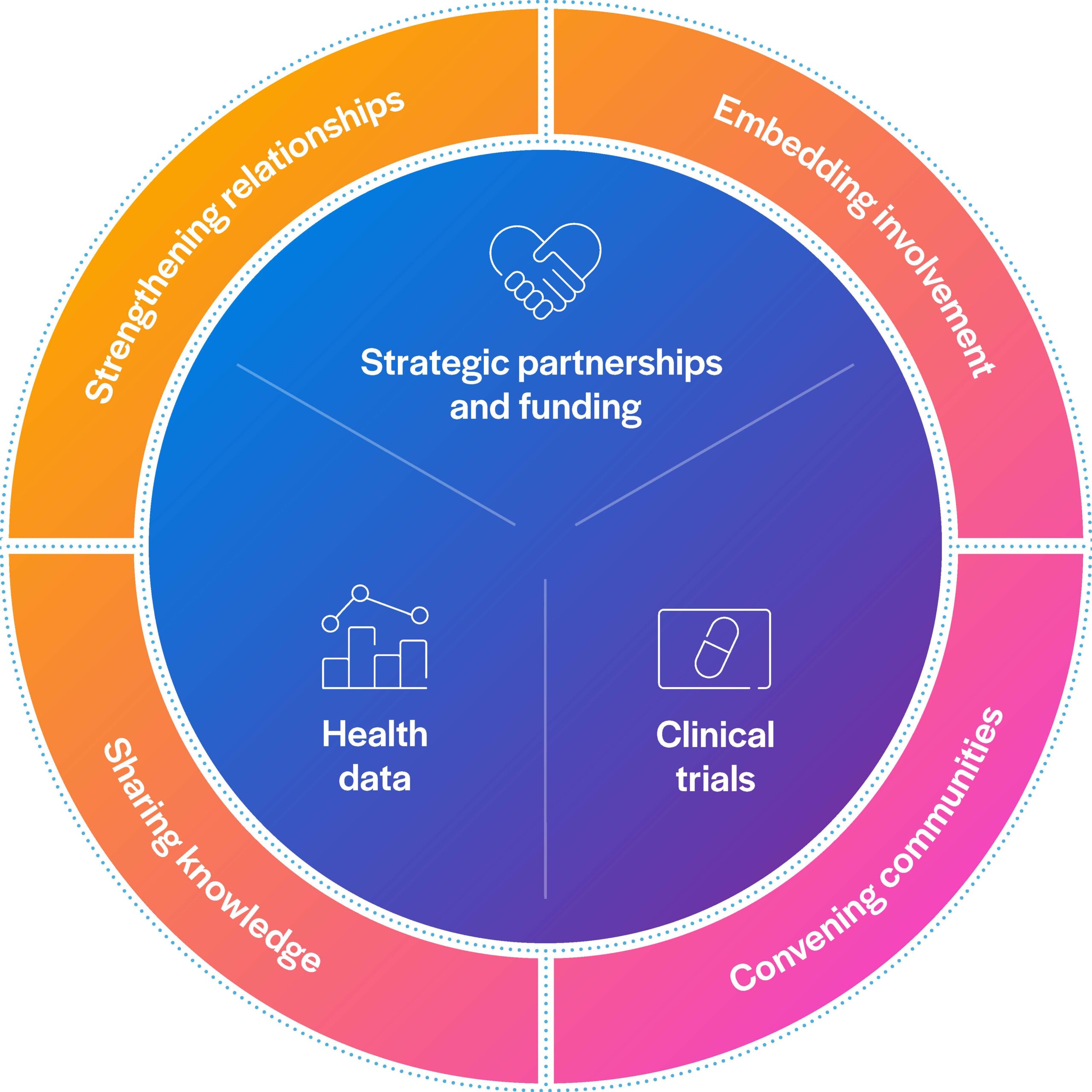
How we work with patients and communities
People with lived experience of health conditions bring unique and valuable knowledge to the research process.
We work with patients and communities, both directly and through our charity partners, to understand unmet needs and identify research priorities.
By involving patients as partners, we can ensure promising scientific discoveries lead to long-lasting impact.

Our patient engagement approach
Patient engagement is essential to improving the quality and impact of health research.
Our patient engagement strategy shares how we work with rare disease and global health patients and communities.
By involving people with lived experience throughout the research process, we will support the development of a more equitable ecosystem where promising science translates into real-world impact.
Through patient engagement, we will:
- confidently invest in research – supporting science with the greatest relevance and potential benefit
- improve clinical trial success – by designing studies that work in real life
- accelerate impact and develop solutions faster – by combining patient experience with scientific and commercial expertise
Our work with people with lived experience
Simple Lung Test Target Product Profile (TPP)
Co-creating faster diagnostic tests for chronic and rare lung conditions

People with lung conditions helped define priorities for a Target Product Profile (TPP) to guide the creation of simple lung tests that can speed up diagnosis of chronic and rare respiratory conditions – such as asthma bronchiectasis, COPD and interstitial lung disease – in primary care.
They shared their diagnostic journeys to highlight what’s most needed in future tests. Insights were gathered from patients, clinicians and healthcare professionals via workshops, surveys and focus groups hosted by LifeArc and Asthma + Lung UK.
The TPP will outline the minimum and desired features for new diagnostic tools. Upon its completion in November 2025, the TPP will be shared with test developers to help produce faster and more accessible diagnostic tests.
Input from people with lived experience will help ensure that new tests meet real-world patient needs.
Translational Innovation Hub Network
Shaping research hubs to transform cystic fibrosis care

People with cystic fibrosis and their families helped shape the vision for research hubs within a national network looking to improve how lung infections are managed.
They shared the biggest challenges in infection diagnosis, treatment and daily care. Their experiences and input were considered when setting up the individual hubs in the network.
These insights also directly influence the scope and focus of funding proposals and chosen projects, and people with cystic fibrosis and their families continue to sit on steering and review groups.
With patient voices involved at every stage, the network will keep its focus on tackling the challenges that matter most to those living with cystic fibrosis.
MND Insights Group
Understanding what people affected by motor neuron disease (MND) want from new drug treatments and technology to help with the challenges of daily life

People with lived experience of MND sit on our MND Insights Group to identify urgent priorities and guide research, programmes and funding decisions.
To date, the group has helped to deliver 8 workshops and 2 community surveys. These activities gathered insights on a wide range of topics about living with MND.
So far, findings have been shared in 2 ‘MND insights reports’. One explores views on treatments, while the other focuses on medical devices and daily living aids.
These perspectives are now being used to inform funding calls, programmes and research design, ensuring scientific progress reflects what truly matters to people with MND and their families.
Drug repurposing funding for MND
Influencing funding decisions to ensure research reflects the needs of people affected by motor neuron disease (MND)

The MND Insights Group shaped a LifeArc-funded, £5 million drug repurposing funding programme for MND.
The group reviewed proposals and shared perspectives on treatment priorities, drug tolerances, feasibility, accessibility and real-world needs.
The set up of the funding call also reflected LifeArc’s patient focus. Applicants were asked to outline clear ‘path to patient’ plans, showing how their research would translate from the lab into the clinic. They also provided lay summaries as part of their submissions.
Involving patients in programmes like this can speed up and prioritise projects that will create effective, tolerable treatments for everyone affected by MND.
C-Further
Working with people affected by children’s and young people’s cancers to guide, influence and shape the work of the consortium

C-Further is advancing new therapies for children and young people with cancer.
A patient advisory panel provides insights and perspectives to the consortium on strategy, prioritisation, policy and communications.
Patient representatives are also involved in reviewing funding applications to the consortium, helping ensure the research funded meets the needs and wants of people affected by children’s and young people’s cancers.
In addition, the consortium works with established patient groups and panels in other organisations to get input from the wider community.
By involving people with lived experience in drug discovery and development efforts, we can ensure research is focused on meaningful patient impact from the earliest stages.
PACE: Pathways to Antimicrobial Clinical Efficacy
Helping to build a scientific portfolio that meets real-world needs

A patient and public advisory group helped to review, shortlist, and select top-ranking antimicrobial resistance research projects for PACE, to build a portfolio that is scientifically strong but meets real-world needs.
Patient and public involvement in translational research
Outlining practical ways to better involve patients and the public in research

This report captures the insights and ideas from a collaborative workshop involving researchers, funders and people with lived experience. It outlines 10 practical actions that can help organisations advance meaningful involvement in translational research.
These include developing best practice guidance, improving communication about translational science, and creating training opportunities for both researchers and people with lived experience.
The workshop was a joint effort between us, the Charities Research Involvement Group (CRIG), and the TAR Network. Attendees shared real-world examples of how patient and public involvement has improved research outcomes.
Our next step is to form a working group to take forward these priorities and support a more inclusive, impactful approach to translational research.
How you can work with us
We partner with patients, families, caregivers, communities and the general public to:
Understand lived experience and identify unmet needs
Prioritise research and guide our funding decisions
Seek insights that inform strategy for our Translational Challenges
Convene multistakeholder discussions with the patient voice at the centre

Working with charities
We also provide advice and support to charities to help accelerate the progression of their funded research.
Who to contact
-

Natasha Ratcliffe
Read bio: Natasha Ratcliffe
Latest news
-
Scientists test whether a finger prick blood test could be used to help diagnose Alzheimer’s disease before symptoms begin
-

Could heart medication or arthritis drugs be used to treat motor neuron disease?
-

Accelerating innovation to transform lung health in primary care
