Michael Dalrymple, Director, Business Development, MRC Technology
The basic idea behind immunotherapy for cancer is to stimulate the body’s immune system to attack a cancer rather than the drug directly targeting the diseased cells. Antibodies such as Herceptin, for example, direct a specific attack on cancerous breast cells but a new generation of antibody drugs can also mediate this immunotherapy approach.
Scientists have discovered that some cancer cells can evade the immune system by locking into the so-called PD-1 (programmed cell death-1) pathway. They avoid destruction by sending the T-Cell part of the immune system a signal that tunes down the ‘attack’ message. An antibody that blocks the PD-1 pathway should therefore prevent this from happening, and the immune system can hunt down and destroy such cancerous cells.
Antibodies are a class of naturally occurring proteins found in the blood which bind to other (usually ‘foreign’) proteins and direct their removal from the body. In 1975 Kohler and Milstein* published a method for creating clonally pure mouse antibody preparations. These ‘monoclonal’ antibodies are exquisitely specific for their target and can be manufactured in huge quantities. Equivalent methods for generating clonal fully human molecules have been less successful, so mouse antibodies are frequently used as a starting point in antibody drug development.
The first therapeutic antibody (Orthoclone OKT3) was approved in 1986, and after a short gap it has been followed by a steady stream of new antibody treatments over the ensuing 29 years. Of the top 10 selling drugs in 2013/14 2 were antibodies (TABLE 1) but there are currently more than 30 FDA (Food and Drug Administration) approved antibody drugs.
Unfortunately, when mouse monoclonal antibodies are used in humans they illicit a so-called HAMA response (human anti-mouse antibody) and the mouse antibodies are quickly destroyed.
The first approach to solving this problem was to create chimæric antibodies where the mouse Fc (Fragment crystallisable) region is replaced with a human Fc. (The Fc region ensures that the antibody binds to a specific receptor, thereby triggering the appropriate immune response). These still create a HAMA response but it is less severe and, under conditions where the immune system is already suppressed, they can be very effective.
In 1986 Greg Winter at the MRC Laboratory of Molecular Biology in Cambridge UK invented a method for ‘humanising’ mouse monoclonals. This works by transplanting the CDRs (sequences in an antibody that interact with the target protein or antigen) from the mouse antibody genes into an equivalent human framework. The resulting genetically engineered antibodies are effectively seen as ‘self’ and the HAMA response is considerably reduced if not eliminated. This CDR grafting approach is the technology behind almost all the earliest therapeutic antibodies and some of the biggest block buster drugs.
The original CDR grafting technology has been slightly improved over the years but the basic principles are still the same. In the wake of CDR grafting’s success, a raft of competing technologies have been invented. These range from what might be regarded as crude variants of CDR grafting to dramatically new ways of producing near or fully human antibodies.
The major competitors to CDR grafting that have emerged over the years include bacteriophage display of fully human antibody fragments, and transgenic mice where the mouse antibody gene repertoire is replaced by the human repertoire.
There have also been significant advances in the use of antibody fragments and various natural and artificial proteins with potent binding properties. However, the tried and trusted CDR grafting technology is still delivering antibody therapeutics almost 30 years after it was first patented.
Of the antibody treatments in Phase III clinical trials 33% have been humanised in this way. This includes the potential new blockbuster Pembrolizumab (now Keytruda®, humanised by MRC Technology in 2008) Keytruda® is an anti-PD-1 antibody and trailblazing the relatively new area of cancer immunotherapy.
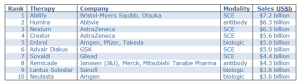
Table 1: World’s top 10 selling drugs in 2013/14
_________
* Köhler, G.; Milstein, C. (1975). “Continuous cultures of fused cells secreting antibody of predefined specificity”. Nature 256 (5517): 495–497